Categoría: STEM (Science, Technology, Engineering and Mathematics)
ORIGINAL
Corrosion Inhibition Performance of Whey Protein-Derived Inhibitors for Low Carbon and Dead Mild Steels in1M Hydrochloric Acid
Inhibición de la corrosión de los inhibidores derivados de la proteína del suero de leche para aceros de bajo carbono y aceros suaves muertos en ácido clorhídrico 1M ácido clorhídrico
Hawraa W. Abd Muslim1 *, Ali Mundher Mustafa1 *, Firas Farhan Sayyid1 *
1Production Eng. and metallurgy. Dept, University of Technology. Baghdad, Iraq.
Cite as: Abd Muslim HW, Mundher Mustafa A, Farhan Sayyid F. Corrosion Inhibition Performance of Whey Protein-Derived Inhibitors for Low Carbon and Dead Mild Steels in1M Hydrochloric Acid. Salud, Ciencia y Tecnología - Serie de Conferencias. 2024; 3:849. https://doi.org/10.56294/sctconf2024849
Submitted: 29-01-2024 Revised: 15-04-2024 Accepted: 04-06-2024 Published: 05-06-2024
Editor:
Dr.
William Castillo-González ![]()
Note: paper presented at the 3rd Annual International Conference on Information & Sciences (AICIS’23).
ABSTRACT
This research investigates the corrosion inhibition capabilities of BCAA-derived inhibitors for low-carbon steels and dead mild carbon steels with distinct carbon contents when exposed to a 1M HCL solution. The effectiveness of the inhibitors was evaluated by measurements of weight loss and polarization. The study revealed that at a concentration of 10 grams, the weighing method showed that the BCAA inhibitor showed protection effectiveness (87 percent) at 313 K for low carbon steel and (89 percent) effectiveness at 303 K for dead carbon steel. Using a concentration of 15 g, the polarization method showed inhibitory activity of (96 percent) at 313 K for low-carbon steel and (96 percent) at 303 K for dead light carbon steel. These results indicate that the inhibition efficiency is affected by the carbon content. Samples were subjected to scanning electron microscopy (SEM) and optical microscope analysis before and after adding the inhibitor. When examined using FTIR spectroscopy, BCAA showed significant efficiency as a corrosion inhibitor for steel alloys immersed in acidic conditions.
Keywords: Corrosion Inhibition; Whey Protein; Low Carbon Steel; Dead Mild Steel; Hydrochloric Acid.
RESUMEN
Esta investigación estudia la capacidad de inhibición de la corrosión de los inhibidores derivados de BCAA para aceros de bajo contenido en carbono y aceros dulces al carbono muertos con distintos contenidos en carbono cuando se exponen a una solución 1M de HCL. La eficacia de los inhibidores se evaluó mediante mediciones de pérdida de peso y polarización. El estudio reveló que, a una concentración de 10 gramos, el método de pesaje mostró que el inhibidor BCAA mostraba una eficacia de protección (87 %) a 313 K para el acero de bajo contenido en carbono y una eficacia (89 %) a 303 K para el acero de carbono muerto. Utilizando una concentración de 15 g, el método de polarización mostró una actividad inhibidora del (96 por ciento) a 313 K para el acero de bajo contenido en carbono y del (96 por ciento) a 303 K para el acero de bajo contenido en carbono muerto. Estos resultados indican que la eficacia inhibidora se ve afectada por el contenido de carbono. Las muestras se sometieron a análisis de microscopía electrónica de barrido (SEM) y microscopía óptica antes y después de añadir el inhibidor. Cuando se examinó mediante espectroscopia FTIR, el BCAA mostró una eficacia significativa como inhibidor de la corrosión de aleaciones de acero sumergidas en condiciones ácidas.
Palabras clave: Inhibición de la Corrosión; Proteína de Suero de Leche; Acero Bajo en Carbono; Acero Dulce Muerto; Ácido Clorhídrico.
INTRODUCTION
Corrosion inhibition is an important practice across industries to mitigate the deterioration of metal surfaces in acidic environments.(1) Inhibitors, both synthetic and derived from natural sources, are widely used for corrosion protection in acidic conditions, especially in processes involving hydrochloric acid such as pickling, descaling, and cleaning.(2) Carbon steel, due to its wide availability, favorable properties, and cost-effectiveness, remains a staple in various sectors. Low carbon steel finds utility in applications such as chemical processing, piping, automotive components, and food can manufacturing.(3) Conversely, dead mild carbon steel, which is characterized by its strength, toughness and malleability, is of interest in diverse applications. Their resistance to stress corrosion cracking makes them valuable in high temperature or corrosive environments. In acidic environments, heterocyclic organic compounds, including HCL and H2SO4, act as corrosion inhibitors by forming protective films on surfaces, a process called preservation.(4) Inhibition mechanisms include complex interactions such as orbital adsorption, chemisorption, and hydrostatic adsorption, which culminate in the creation of a barrier layer that prevents corrosive agents from reaching the metal surface.(5) Molecular features such as functional groups, steric barriers, and aromaticity, along with electronic structure properties, influence adsorption, which depends on electron donation, density, and structure.(6) The effectiveness of corrosion inhibition is determined by the adsorption capacity and properties of the layer formed in the specific environment.(7) Heterocyclic organic molecules containing heteroatoms (S, N, and O) and pi electrons can act as corrosion inhibitors in mild steel. This study aims to investigate the potential of expired BCAA (Leucine, Isoleucine, and Valine) as a corrosion inhibitor for steel alloys in an HCL solution. BCAA, which is a blend of essential amino acids, is well-known for its role in muscle protein synthesis and recovery, making it a popular nutritional supplement in the fitness industry. The novelty of this research lies in exploring the use of BCAA as a corrosion inhibitor, which offers several advantages. Firstly, BCAA is easily accessible, cost-effective, and environmentally friendly, aligning with the increasing demand for sustainable alternatives in various industries. Secondly, the presence of functional groups like amino and carboxyl groups in BCAA molecules suggests potential interactions with the metal surface, leading to the formation of protective films.(8) Protein identification was performed using FTIR spectroscopy, and the corrosion inhibition effectiveness of whey protein for low-carbon steel in 1M HCL solution was evaluated within a temperature range of 303 K to 333 K.(9) Dynamic polarization and weight loss techniques were used. For evaluation. The primary objective of this research is to study the corrosion inhibition potential of whey protein derived inhibitors when applied to low carbon steel and dead carbon steel in a 1M HCL environment.(10) By exploring the effectiveness of these inhibitors on steels with different carbon contents, this study aims to provide insight into their performance and suitability for corrosion protection applications.(11)
This study presents a new approach through the use of whey protein-derived inhibitors to address corrosion challenges in premium carbon steel alloys. Examination of both low carbon steels and dead light carbon steels confirms the ability of these inhibitors to meet various industrial requirements. The use of these protein-derived inhibitors represents an innovative approach to environmentally friendly and sustainable corrosion protection solutions.(12)
The aim of the research
Evaluation of the corrosion inhibition efficiency of whey protein derived inhibitors on low carbon steel and dead mild carbon steel in 1M HCL solution. Comparing the effectiveness of inhibitors on low carbon and dead carbon steel by evaluating their corrosion rates using weight loss and polarization measurements and studying the effect of inhibitor concentration, immersion time, and temperature on the corrosion protection performance of inhibitors derived from whey protein. Clarifying the mechanism of adsorption of inhibitors on steel surfaces using FTIR spectroscopy. Contributing to understanding the relationship between carbon content and the effectiveness of corrosion inhibitors, thus enhancing knowledge of corrosion protection strategies for different steel alloys.
METHOD
Corrosion Solution Preparation
To formulate the corrosion solution, a mixture of hydrochloric acid and distilled water was blended at a ratio of 37 % acid to 63 % water. The resultant solution, totaling 1000 ml, encompassed inhibitor concentrations of 10g and 15g.(13,14)
Metal Alloy Selection
For the experimental investigation, specimens of low-carbon steel (Table 2) and dead mild carbon steel (Table 3), measuring 2 cm by 4 cm, were chosen. These samples underwent analysis through an Optical Emission Spectrometer (OES) at Al-Nabaa Engineering Services Company to facilitate weight loss and polarization assessments.
Weight Loss Method
The gravimetric weight loss method was employed to quantify the corrosion rate. Samples of low-carbon and dead-carbon steel were placed in reaction vessels containing 1M HCL solution with varying inhibitor concentrations. Prior to immersion, each sample’s weight was determined using a solid support system measuring 2 cm by 4 cm. Immersion durations ranged from 2 to 24 hours, across temperatures of 303, 313, 323, and 333 K. After removal of the corrosive medium and thorough elimination of corrosion products, the metal specimens were re-weighed. The corrosion rate (CR) and corrosion efficiency (%) were calculated using Equations (1) and (2).(15,16) incorporating average mass loss (W), steel density (ρ), surface area (a), and exposure time (t).
![]()
Equation (1) is used to calculate the corrosion rate (CR), with factors including average mass loss (W), steel density (ρ), surface area (a), and Exposure time (t).
![]()
Equation (2) is utilized to compute the inhibition efficiency of the tested inhibitor.
This equation compares the rate of corrosion without the inhibitor (CR0) to the rate of corrosion with the inhibitor (CR).
Polarization Technique
To ensure that only the surface area is exposed to the solution, the working electrode in a corrosion cell is a low and dead carbon steel sample set on a platform with a 1 cm2 area hole, In this study, the reference electrode was a saturated calomel electrode (SCE) bridged by a Laggin-Haber probe; it was positioned 1 mm distant from the solution-facing side of the working electrode to reduce experimental error caused by low IR drop. The platinum electrochemical cell directly opposite the working electrode. As depicted in figure 1, polarization was performed in a 1-liter beaker containing working, counter, and reference electrodes, which was placed on a mental heater with a magnetic stirrer (type Heidolph, MR Hei Standard / W-Germany) to heat the solution to the desired temperature and rotate it at the required speed, which varies for each experiment. Using the Potentiostat (LIDA INSTRUMENT-Germany) depicted in figure 1, constant potentials were applied to the sample in the anodic and cathodic areas.(3) This Potentiostat can provide consistent potentials between -1 V and 1 V, the standard calomel electrode potential (SCE). The investigated measurement potential range for open circuit potential was between (-1,2) and (+3) mV. (OCP). The Scan rate, which is 2mV/s, determines the maximum sweep speed. The potential difference between the working and reference electrodes was (WE - RE), and any current flowing through the working electrode and auxiliary electrode could be monitored and automatically recorded using computer software.
Fourier Transform Infrared (FTIR) Analysis
One important test was Fourier transform infrared spectroscopy (FTIR). It is an analytical technique largely used to determine the presence of specific covalent bands from organic materials, as well as in some cases bands found in inorganic materials. Since this test was very sensitive for different functional groups, the presence of small amounts of polymers was very efficiently detected. In this technique, the absorption of different wavelengths of infrared radiation by the substance of interest is measured. FTIR spectrometers can simultaneously collect data in a wide spectral range and provide raw data the advantage over a dispersive spectrometer is that it measures intensity in a narrow range of wavelengths at the same time. These wave-absorption bonds define specific molecular components as well as structures. FTIR has been applied to determine the presence of specific covalent bonds before and after the decomposition or mineralization process in simulated body fluids. FTIR is very sensitive to vibrations of functional groups. Therefore, it is very effective for detecting the presence of small amounts of polymers and presenting molecular-level polymer interactions and HA mineralization. This test was conducted at the Center for Nanotechnology and Advanced Materials, University of Technology, Baghdad, Iraq.
RESULTS
Weight Loss Measurements
Figure 1 illustrates the corrosion rates and inhibition efficiencies observed for low carbon steels, while figure 2 presents the corresponding data for dead mild carbon steels. These measurements were conducted in a 1 M HCL solution without the presence of any inhibitor. Our findings indicate substantial corrosion on the metal surfaces across varying temperatures and immersion durations.(17,18) In figure 3, the impact of adding 10 grams of the inhibitor concentrate (WP) to an alloy with a carbon content of 0,06 is depicted, while figure 4 portrays the effect when the alloy contains a lower carbon content of 0,019. These graphical representations reveal the influence of the inhibitor under different conditions. Furthermore, figures 5 and 6 showcase notable reductions in corrosion rates and simultaneous enhancements in protection efficiency when a 15-gram concentration of the inhibitor (WP) is introduced under equivalent temperature and duration variations.(19,20) To expand on these weight loss measurements, we conducted an in-depth analysis of the corrosion rate data and inhibition efficiencies. The following subsections provide a detailed examination of the results obtained for both low carbon and dead mild carbon steels under various experimental conditions.

Figure 1. The impact of temperature on corrosion rate without the inhibitor of low carbon steel

Figure 2. Effect of adding 10g of inhibitor at various temperatures for low carbon steel
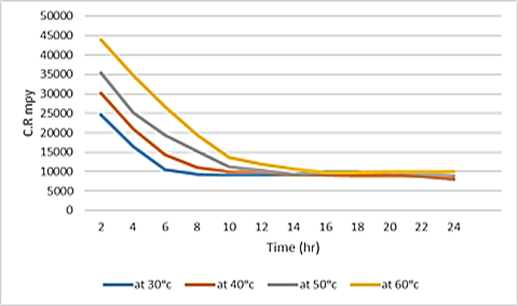
Figure 3. Impacts of 15 g of inhibitor at different temperature secularly for low carbon steel
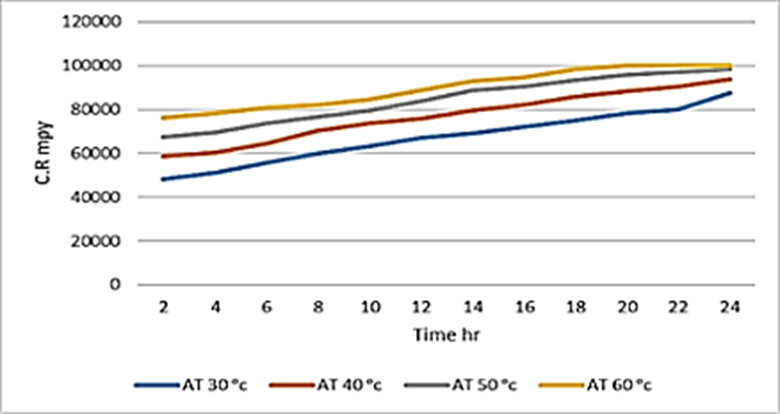
Figure 4. Correlation between temperature and Corrosion rate over time without any inhibitor of dead mild carbon steel
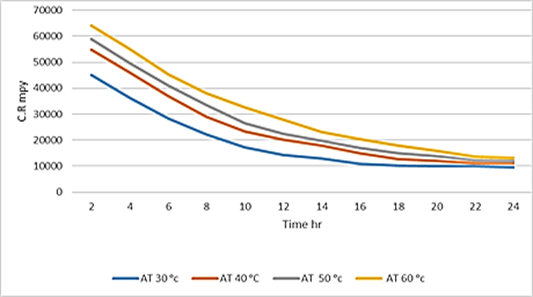
Figure 5. Variation in corrosion rate over time at different temperatures when 10 g of inhibitor is added of dead mild carbon steel

Figure 6. Relationship between corrosion rate and time at various temperatures with the inclusion of 15 g of inhibitor of dead mild carbon steel
Influence of Temperature on Corrosion and Inhibition
Figures 7 and 8 provide crucial insights into the impact of temperature on the corrosion rate and the effectiveness of corrosion inhibition within a 1 M HCL solution, considering various alloys and different inhibitor concentrations. Our assessment revealed that an immersion period of 2 hours emerged as the optimal duration, a choice informed by a meticulous analysis of inhibitory efficiency across multiple concentrations. These figures unveil a significant trend: as the temperature escalated within the range of 303 to 333 K, the protective efficiency showed a noticeable decline. This intriguing temperature-dependent behavior warrants deeper exploration. The mechanism of action of the inhibitor involves adsorption onto the steel surface. At higher temperatures, an intriguing phenomenon comes into play: there’s a heightened likelihood of inhibitor molecules desorbing from the steel substrate.(21,22) This phenomenon may be attributed to the increased kinetic energy of the molecules at elevated temperatures, which can disrupt the adsorption equilibrium on the metal surface. The observed decrease in protective efficiency with rising temperatures emphasizes the critical role of temperature control in corrosion inhibition strategies. It highlights the necessity of optimizing temperature conditions to ensure the sustained adsorption of inhibitors on the steel surface. The implications of this temperature-dependent behavior are further dissected in the subsequent sections of our analysis.

Figure 7. Presents an analysis of weight loss for low alloy steels, focusing on low carbon steel, across different temperatures (303-333 K) and inhibitor concentrations

Figure 8. Displays the results of weight loss analysis for dead mild carbon steel at various temperatures (303-333 K) and inhibitor concentrations
Corrosion Inhibition Effect
However, the narrative changes significantly when different concentrations of the inhibitor are introduced. Remarkably, the addition of this inhibitor leads to a tangible reduction in corrosion rates at the same range of temperatures. Notably, the data shows that the introduction of 15 grams of the inhibitor brings about a more substantial decrease in corrosion rates when compared to adding 10 grams of the inhibitor for both alloy types. This finding underscores a pivotal point: the effectiveness of corrosion protection increases with the concentration of the inhibitor. The direct correlation between inhibitor concentration and corrosion rate reduction underscores the practical importance of optimizing inhibitor dosages for enhanced corrosion resistance.(23,24) These results illuminate the potential of the whey protein-derived inhibitor in mitigating corrosion in acidic environments. The next sections further dissect these findings and explore the underlying mechanisms that govern the inhibitor’s effectiveness.

Figure 9. Compares corrosion rates at different temperatures for low carbon steel, both with and without the inclusion of the inhibitor at varying concentrations

Figure 10. Examines the correlation between inhibitor concentration, corrosion rates, and different temperatures for dead mild steel, both in the absence and presence of the inhibitor
DISCUSSION
Open Circuit Potential (OCP) Analysis
An essential parameter for evaluating corrosion behavior is the measurement of the open circuit potential (OCP) over time, which offers valuable insights into the free corrosion potential of both uninhibited and inhibited samples. When a sample is immersed in the electrolyte, the initial potential is promptly recorded and continuously monitored over time, referencing the standard calomel electrode (SCE). The results obtained from these measurements, conducted across varying inhibitor concentrations, reveal a discernible pattern. One noteworthy observation is the temperature’s significant influence on the kinetics of the reaction (Figures 11-16). As the temperature increases, the reaction rate accelerates, resulting in a gradual decline in inactivity over time. Consequently, the state of equilibrium is not protracted; rather, it diminishes relatively quickly. This behavior highlights the dynamic nature of corrosion processes, particularly in the context of inhibitor presence and temperature variations. It suggests that higher temperatures can lead to more rapid corrosion reactions, potentially compromising the effectiveness of inhibitors. The intricate interplay between temperature, reaction kinetics, and inhibitor performance warrants further examination. In the subsequent sections, we delve deeper into the implications of these findings and explore their significance for designing effective corrosion protection strategies.

Figure 11. Without inhibitor for Dead mild steel

Figure 12. With (10 g) inhibitor

Figure 13. With (15 g) inhibitor

Figure 14. Without inhibitor for low carbon steel

Figure 15. With (10 g) inhibitor for low carbon

Figure 16. With (15 g) inhibitor
Cyclic Re passivation
The refractory process involves the study of the kinetics of film coating and anodic processes that impact the changing environment and coating conditions. When a solution undergoing dissociation is sufficiently closed to maintain a distinct composition separate from the bulk, we must consider the implications of these compositional changes. As a result, anodic current transients need to be distinguished between components related to film coverage and those associated with metal melting. The figures below indicate that the corrosion rate is higher at 30 degrees Celsius compared to 40 degrees Celsius, and the level of protection at 40 degrees Celsius is greater than at higher temperatures, around 50-60 degrees Celsius. This is because these temperatures are closer to equilibrium, which can lead to the formation of corrosion pits. When small amounts (10 and 15 grams) of inhibitors are added, the cyclic process only occurs at a temperature of 30°C. This is primarily due to the higher temperatures encountered when exposed to elevated temperatures, these inhibitors can oxidize or evaporate more rapidly, reducing their ability to protect surfaces from corrosion. Additionally, temperature can influence the chemical reactions that take place between the inhibitor and the metal surface. Under specific conditions, the interaction between the inhibitor and the metal at high temperatures can result in the formation of a surface layer. While this layer may offer some benefits, it can also lead to undesirable reactions or prove ineffective in safeguarding the metal surface. Moreover, temperature fluctuations can weaken the bonds between the corrosion inhibitor and the surface, diminishing the overall efficiency of the inhibitor in providing protection against corrosion.
|
Table 1. Indices corrosion parameter for low carbon steel before inhibition |
||||||
|
ITEM |
E corr.(volt) |
I corr.(Amp) |
Corr. Rate mmpy |
βa |
βc |
OCP (volt) |
|
30ᵒC |
-0,428 |
5,539*10-4 |
3,113 |
0,117 |
0,160 |
-0,4709 |
|
40ᵒC |
-0,442 |
5,586*10-3 |
31,298 |
0,145 |
0,143 |
-0,4734 |
|
50ᵒC |
-0,404 |
1,764*10-2 |
99,156 |
0,092 |
0,177 |
-0,4619 |
|
60ᵒC |
-0,443 |
8,066*10-2 |
453,400 |
0,174 |
0,180 |
-0,4785 |
|
Table 2. Indices corrosion parameter for low carbon steel in 10 g inhibition |
||||||
|
ITEM |
E corr.(volt) |
I corr.(Amp) |
Corr. Rate mmpy |
βa |
βc |
OCP (volt) |
|
30ᵒC |
-0,457 |
1,136*10-4 |
0,638 |
0,153 |
0,175 |
-0,3276 |
|
40ᵒC |
-0,448 |
4,967*10-4 |
2,792 |
0,087 |
0,134 |
-0,459 |
|
50ᵒC |
-0,427 |
7,504*10-3 |
42,180 |
0,138 |
0,105 |
-0,4670 |
|
60ᵒC |
-0,451 |
1,874*10-2 |
105,399 |
0,136 |
0,131 |
-0,5207 |
|
Table 3. Indices corrosion parameter for low carbon steel in 15 g inhibition |
||||||
|
ITEM |
E corr.(volt) |
I corr. (Amp) |
Corr. Rate mmpy |
βa |
βc |
OCP (volt) |
|
30ᵒC |
-0,585 |
9,272*10-5 |
0,521 |
0,238 |
0,070 |
-0,472 |
|
40ᵒC |
-0,527 |
1,845*10-4 |
1,037 |
0,187 |
0,087 |
-0,509 |
|
50ᵒC |
-0,577 |
1,397*10-3 |
7,852 |
0,201 |
0,157 |
-0,579 |
|
60ᵒC |
-0,556 |
7,453*10-3 |
41,894 |
0,233 |
0,192 |
-0,587 |

Figure 17. Before inhibitor

Figure 18. With 10 g inhibitor
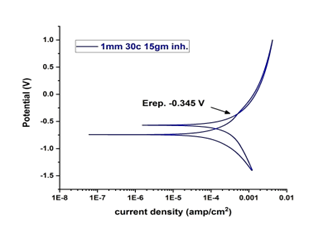
Figure 19. With 15 g inhibitor
Fourier transform infrared (FTIR) analysis
Analysis of whey protein by Fourier transform infrared (FTIR) is shown in figure 20. FTIR spectroscopy covered a transmittance range from 500 to 4000 cm-1. The examination was performed using a German-made BRUKER ABB/SPECTROLAP Fourier Transform Infrared (FTIR) device. Bonds such as primary amines, methylene’s, aromatic bonds, primary alcohols, and tertiary alcohols were identified in the FTIR spectrum of the protein. These bonds (N-H, C-H, C-C, CO, C=C, C-X) contributed to the barrier layer, giving protection to the steel surface. The chemical structure of the inhibitor is denoted by NH2CH COOH.

Figure 20. Inhibitor analysis by FTIR method
Optical Microscopic Analysis
Microstructural Examination
In the realm of corrosion analysis, a pivotal aspect involves the study of microstructural changes in carbon steel alloy samples, both prior to and following the introduction of corrosion inhibitors via the weight loss method.
Microstructure - Weight Loss Method
Figure 21 presents an optical micrograph that vividly illustrates the consequences of exposing a low carbon steel alloy sample to a corrosive medium. Dark regions within the micrograph clearly indicate corrosion attacks, showcasing the diverse effects experienced under varying environmental conditions. Contrastingly, figure 22 provides a compelling visual narrative of the protective effect engendered by the addition of an inhibitor to the corrosive medium. In this scenario, the inhibitor displays high absorption characteristics, manifesting as a discernible protective layer enveloping the metal surface. This transformation exemplifies the inhibitor’s role in mitigating corrosion-induced damage.

Figure 21. Without inhibitor

Figure 22. With inhibitor
Microstructure Before and After Polarization Method
Beyond the weight loss method, a parallel examination was conducted utilizing the polarization method to investigate the microstructure of carbon steel alloy samples. Figure 23 presents an optical micrograph captured before the introduction of the inhibitor. This image provides a baseline perspective of the initial microstructural state of the alloy.

Figure 23. Without inhibitor

Figure 24. With inhibitor
In stark contrast, figure 24 depicts the microstructure following the introduction of the inhibitor, obtained through the polarization method. The transformation is palpable, emphasizing the potential of the inhibitor to induce protective changes in the alloy’s microstructure. These optical micrographs underscore the dynamic interplay between corrosion and inhibition, offering tangible visual evidence of the inhibitor’s impact on microstructural integrity. The implications of these findings are further explored in the subsequent sections, shedding light on their broader significance for corrosion management strategies.
Analysis using Scanning Electron Microscope (SEM)
Figure 25 shows SEM micrographs of a corroded and protected low-and dead carbon steel sample. In figure 25, the metal is seen in an intermediate stage of corrosion without the presence of an inhibitor, where the dark spots indicate corrosion attack. Figures 26 and 27 illustrates the scenario where an inhibitor is added to the acidic solution. With both methods, the high absorption of the inhibitor forms a protective coating on the metal surface, providing clear protection. The adsorption of the inhibitor involves the formation of acceptor-donor bonds between the unpaired electrons of the heteroatoms in the inhibitor and the active centers of the metal surface. Previous studies have shown similarities between the behavior of uncoated and inhibited solutions and the carbon annealing process. Organic compound adsorption can be classified into two main types: physical adsorption and chemisorption. Physical adsorption occurs when there is an electrically charged surface on the metal and charged species in the surrounding solution. On the other hand, chemisorption involves the sharing or transfer of charges between the inhibitor molecules and the metal surface, leading to the formation of a coordinate bond. This type of adsorption can occur on both positively and negatively charged surfaces. For chemisorption to occur, a transition metal with vacant low-energy electron orbitals and an inhibitor with molecules possessing loosely bound electrons or heteroatoms with lone pairs of electrons are necessary.(25)
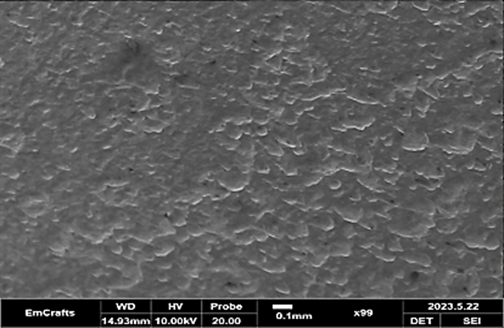
Figure 25. Base metal

Figure 26. After adding the inhibitor in a way to lose weight

Figure 27. After adding the inhibitor by Polarization method
CONCLUSIONS
In light of the comprehensive investigation conducted, several key conclusions emerge, shedding valuable insights into the corrosion behavior of carbon steel alloys and the effectiveness of whey-derived inhibitors:
· Carbon Content and Corrosion Inhibition: weight loss analysis elucidated that the addition of 10 grams of inhibitor at 313 K yielded the highest corrosion inhibition efficiency, reaching 87 % for low carbon steel. Similarly, at 303 K, a concentration of 10 grams achieved a noteworthy corrosion inhibition efficiency of 89 %. These findings, tethered to the carbon content of the alloys, suggest a direct correlation: lower carbon content corresponds to enhanced barrier efficiency.
· Temperature-Driven Corrosion: notably, corrosion rates displayed an upward trajectory with increasing temperature, regardless of the presence or absence of corrosion inhibitors. This temperature-driven acceleration underscores the complex dynamics of corrosion kinetics.
· Temperature and Inhibition Efficiency: as temperature escalated from 303 to 333 K, a concurrent decrease in protection efficiency was observed. This decline can be ascribed to the reduced adsorption of the inhibitor onto the steel surface, emphasizing the significance of temperature control in inhibitor applications.
· Impact of Inhibitor on Corrosion Rates: polarization analysis unveiled elevated corrosion rates on the metal surface across various temperatures. However, the introduction of different inhibitor concentrations led to a discernible reduction in corrosion rates, underlining the inhibitor’s potential in mitigating corrosion.
· Dose-Dependent Efficacy: with an increase in inhibitor dosage to 15 grams, the maximum corrosion inhibition efficiency surged to 96 % at 313 k for low carbon steel and 96 % at 303 k for dead carbon steel. intriguingly, corrosion rates exhibited a more significant reduction when 15 grams of the inhibitor were added compared to the addition of 10 grams for both alloy types.
· Whey Protein as an Effective Inhibitor: collectively, these results unmistakably affirm that whey protein serves as a highly effective inhibitor, providing robust protection to both alloys against corrosion in acidic solutions.
REFERENCES
1. Mustafa, A. M., F. F. Sayyid, N. Betti, L. M. Shaker, M. M. Hanoon, A. A. Alamiery, A. A. H. Kadhum, and M. S. Takriff. “Inhibition of mild steel corrosion in hydrochloric acid environment by 1-amino-2-mercapto-5-(4-(pyrrol-1-yl) phenyl)-1, 3, 4-triazole.” South African Journal of Chemical Engineering 39, no. 1 (2022): 42-51.
2. Jawad, D. Zinad, R.D. Salim, A.A. Al-Amiery, T.S. Gaaz, M.S. Takriff and A. Kadhum, Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment, Coatings, 2019, 9, 729. doi: 10.3390/coatings9110729
3. S. Al-Baghdadi, F. Noori, W.K. Ahmed and A.A. Al-Amiery, Thiadiazole as a potential corrosion inhibitor for mild steel in 1 M HCl, J. Adv. Electrochem., 2016, 2, 67–69.
4. Mustafa, A. M., Z. S. Abdullahe, F. F. Sayyid, M. M. Hanoon, A. A. Al-Amiery, and W. N. R. W. Isahak. “3-Nitrobenzaldehyde-4-phenylthiosemicarbazone as Active Corrosion Inhibitor for Mild Steel in a Hydrochloric Acid Environment.” Progress in Color, Colorants and Coatings 15, no. 4 (2022): 285-293.
5. S. Junaedi, A. Al-Amiery, A. Kadihum, A. Kadhum and A. Mohamad, Inhibition effects of a synthesized novel 4-aminoantipyrine derivative on the corrosion of mild steel in hydrochloric acid solution together with quantum chemicalstudies, Int. J. Mol. Sci., 2013, 14, 11915–11928. doi: 10.3390/ijms140611915
6. A. Alamiery, W.N.R.W. Isahak, H.S.S. Aljibori, H.A. Al-Asadi and A.A.H. Kadhum, Effect of the structure, immersion time and temperature on the corrosion inhibition of 4- pyrrol-1-yl-n-(2,5-dimethyl-pyrrol-1-yl)benzoyl amine in 1.0 M HCl solution, Int. J. Corros. Scale Inhib. 2021, 10, no. 2, 700–713. doi: 10.17675/2305-6894-2021-10-2-14
7. W.K. Al-Azzawi, A.J. Al Adily, F.F. Sayyid, R.K. Al-Azzawi, M.H. Kzar, H.N. Jawoosh, A.A. Al-Amiery, A.A.H. Kadhum, W.N.R.W. Isahak and M.S. Takriff, Evaluation of corrosion inhibition characteristics of an N-propionanilide derivative for mild steel in 1 M HCl: Gravimetrical and computational studies, Int. J. Corros. Scale Inhib. 2022, 11, no. 3, 1100–1114. doi: 10.17675/2305-6894-2022-11-3-12
8. Mohamed, M. Taha, S. A. Nawi, A. M. Mustafa, F. F. Sayyid, M. M. Hanoon, A. A. Al-Amiery, A. A. H. Kadhum, and W. K. Al-Azzawi. “Revolutionizing Corrosion Defense: Unlocking the Power of Expired BCAA.”
9. Y.M. Abdulsahib, A.J.M. Eltmimi, S.A. Alphabet, M.M. Hanoon, A.A. Al-Amiery, T. Allami and A.A.H. Kadhum, Experimental and theoretical investigations on the inhibition efficiency of N-(2,4-dihydroxytolueneylidene)-4-methylpyridin-2-amine for the corrosion of mild steel in hydrochloric acid, Int. J. Corros. Scale Inhib. 2021, 10, no. 3, 885–899. doi: 10.17675/2305-6894-2021-10-3-3
10. A.K. Khudhair, A.M. Mustafa, M.M. Hanoon, A. Al-Amiery, L.M. Shaker, T. Gazz, A.B. Mohamad, A.H. Kadhum and M.S. Takriff, Experimental and Theoretical Investigation on the Corrosion Inhibitor Potential of N-MEH for Mild Steel in HCl, Prog. Color, Color. Coat. 2022, 15, 111–122. doi: 10.30509/PCCC.2021.166815.1111
11. D.S. Zinad, R.D. Salim, N. Betti, L.M. Shaker and A.A. AL-Amiery, Comparative Investigations of the Corrosion Inhibition Efficiency of a 1-phenyl-2-(1- phenylethylidene)hydrazine and its Analog Against Mild Steel Corrosion in Hydrochloric Acid Solution, Prog. Color, Color. Coat. 2022, 15, 53–63. doi: 10.30509/pccc.2021.166786.1108
12. R.D. Salim, N. Betti, M. Hanoon and A.A. Al-Amiery, 2-(2, 4-Dimethoxybenzylidene)- N-Phenylhydrazinecarbothioamide as an Efficient Corrosion Inhibitor for Mild Steel in Acidic Environment, Prog. Color, Color. Coat. 2021, 15, 45–52. Doi: 10.30509/pccc.2021.166775.1105.
13. A. Mustafa, F. Sayyid, N. Betti, M. Hanoon, A. Al-Amiery, A. Kadhum and M. Takriff, Inhibition Evaluation of 5-(4-(1H-pyrrol-1-yl)phenyl)-2-mercapto-1,3,4-oxadiazole for the Corrosion of Mild Steel in an Acid environment: Thermodynamic and DFT Aspects, Tribologia, 2021, 38, 39–47. doi: 10.30678/fjt.105330
14. A. Alamiery, W.N.R.W. Isahak, H. Aljibori, H. Al-Asadi and A. Kadhum, Effect of the structure, immersion time and temperature on the corrosion inhibition of 4-pyrrol-1-yln- (2,5-dimethyl-pyrrol-1-yl)benzoylamine in 1.0 m HCl solution, Int. J. Corros. Scale Inhib. 2021, 10, 700–713. doi: 10.17675/2305-6894-2021-10-2-14
15. A. Alamiery, E. Mahmoudi and T. Allami, Corrosion inhibition of low-carbon steel in hydrochloric acid environment using a Schiff base derived from pyrrole: gravimetric and computational studies, Int. J. Corros. Scale Inhib. 2021, 10, 749–765. doi: 10.17675/2305-6894-2021-10-2-17
16. Sayyid, Firas F., Ali M. Mustafa, Mahdi M. Hanoon, Lina M. Shaker, and Ahmed A. Alamiery. “Corrosion Protection Effectiveness and Adsorption Performance of Schiff Base-Quinazoline on Mild Steel in HCl Environment.” Corrosion Science and Technology 21, no. 2 (2022): 77-88.
17. Mustafa, A. M., F. F. Sayyid, N. Betti, M. M. Hanoon, Ahmed Al-Amiery, A. A. H. Kadhum, and M. S. Takriff. “Inhibition Evaluation of 5-(4-(1H-pyrrol-1-yl) phenyl)-2-mercapto-1, 3, 4-oxadiazole for the Corrosion of Mild Steel in an Acidic Environment: Thermodynamic and DFT Aspects.” Tribologia-Finnish Journal of Tribology 38, no. 3− 4 (2021): 39-47.
18. A.Z. Salman, Q.A. Jawad, K.S. Ridah, L.M. Shaker and A.A. Al-Amiery, Selected BISThiadiazole: Synthesis and Corrosion Inhibition Studies on Mild Steel in HCL Environment, Surf. Rev. Lett., 2020, 27, 2050014. doi: 10.1142/S0218625X20500146
19. A. Fouda, A. Al-Sarawy and E. El-Katori, Thiazole derivatives as corrosion inhibitors for C-steel in sulphuric acid solution, Eur. J. Chem., 2010, 1, no. 4, 312–318. doi: 10.5155/eurjchem.1.4.312-318.105
20. Sayyid, Firas F., Ali M. Mustafa, Mahdi M. Hanoon, Lina M. Shaker, and Ahmed A. Alamiery. “Corrosion Protection Effectiveness and Adsorption Performance of Schiff Base-Quinazoline on Mild Steel in HCl Environment.” Corrosion Science and Technology 21, no. 2 (2022): 77-88.
21. S. Al-Baghdadi, A. Al-Amiery, T. Gaaz and A. Kadhum, Terephthalohydrazide and isophthalo-hydrazide as new corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical approaches, Koroze Ochre. Mater. 2021, 65, 12–22. doi: 10.2478/kom-2021-0002
22. Abbas, Ahmed S., Bahaa Sami Mahdi, Haider H. Abbas, F. F. Sayyid, A. M. Mustafa, Iman Adnan Annon, Yasir Muhi Abdulsahib, A. M. Resen, M. M. Hanoon, and Nareen Hafidh Obaeed. “Corrosion Behavior Optimization by Nanocoating Layer for Low Carbon Steel in Acid and Salt Media.” CORROSION SCIENCE AND TECHNOLOGY 22, no. 1 (2023): 1-9. 23.S.Junaedi, A.A.H. Kadhum, A. Al-Amiery, A.B. Mohamad and M.S. Takriff, Synthesis and characterization of novel corrosion inhibitor derived from oleic acid: 2-Amino-5- Oleyl 1,3,4-Thiadiazol (AOT), Int. J. Electrochem. Sci., 2012, 7, 3543–3554. doi: 10.1016/S1452-3981(23)13976-9
23. S. Al-Baghdadi, F. Hashim, A. Salam, T. Abed, T. Gaaz, A. Al-Amiery, A.H. Kadhum, K. Reda and W. Ahmed, Synthesis and corrosion inhibition application of NATN on mild steel surface in acidic media complemented with DFT studies, Results Phys., 2018, 8, 1178–1184. Doe: 10.1016/j.rinp.2018.02.007.
24. Al-Azzawi, W. K., A. J. Al Adily, F. F. Sayyid, R. K. Al-Azzawi, M. H. Kzar, H. N. Jawoosh, A. A. Al-Amiery, A. A. H. Kadhum, W. N. R. W. Isahak, and M. S. Takriff. “Evaluation of corrosion inhibition characteristics of an N-propionanilide derivative for mild steel in 1 M HCl: Gravimetrical and computational studies.” Int. J. Corros. Scale Inhib 11, no. 3 (2022): 1100-1114.
25. Abbass, M. K., K. M. Raheef, I. A. Aziz, M. M. Hanoon, A. M. Mustafa, W. K. Al-Azzawi, A. A. Al-Amiery, and A. A. H. Kadhum. “Evaluation of 2-Dimethylaminopropionamidoantipyrine as a Corrosion Inhibitor for Mild Steel in HCl Solution: A Combined Experimental and Theoretical Study.” Progress in Color, Colorants and Coatings 17, no. 1 (2024): 1-10.
26. Khudhair, A. K., A. M. Mustafa, M. M. Hanoon, A. Al-Amiery, L. M. Shaker, T. Gazz, A. B. Mohamad, A. H. Kadhum, and M. S. Takriff. “Experimental and theoretical investigation on the corrosion inhibitor potential of N-MEH for mild steel in HCl.” Progress in Color, Colorants and Coatings 15, no. 2 (2022): 111-122.
27. F. F. Sayyied. Et al. (2021), studied the Corrosion Protection Effectiveness and Adsorption Performance of Schiff Base-Quinazoline on Mild Steel in HCL Environment.
28. Sayyid, Firas F., Slafa I. Ibrahim, Mahdi M. Hanoon, A. A. H. Kadhum, and A. A. Al-amiery. “Gravimetric Measurements and Theoretical Calculations of 4-Aminoantipyrine Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution: Comparative Studies.” Corrosion Science and Technology 22, no. 2 (2023): 73-89.
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.
Data curation: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.
Formal analysis: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.
Research: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.
Methodology: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.
Drafting - original draft: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.
Writing - proofreading and editing: Hawraa W. Abd Muslim, Ali Mundher Mustafa, Firas Farhan Sayyid.